The SAnDMAN study Sedation, Analgesia and Delirium MANagement
What?
SANDMAN is an observational study that will describe sedation, analgesia, and delirium strategies used in ICUs around the world.
The study is endorsed by the ESICM Health Services Research & Outcome (HSRO), Neuro-Intensive Care (NIC) and Peri-Operative Intensive Care (POIC) sections.
SAnDMAN received an ESICM 2018 trials group award.
Why?
Pain and sedation management has a significant impact on short-term and long-term outcomes of critically ill patients. Despite strong evidence and international guidelines, there is tremendous variability in management internationally. It is vital to understand current practices around the world, however there are no large-scale multinational data describing sedation, analgesia and delirium practices in the intensive care unit (ICU).
Design: Retrospective study to describe patterns of sedative, analgesic, and antipsychotic drug use and adherence to evidence-based strategies and guidelines. We will collect data in upper and lower/middle income countries, academic and non-academic ICUs, and on heterogeneous populations of critically ill patients.
Sample Size: We aim to recruit more than 2000 patients internationally from a minimum of 100 ICUs representing geographic and socio-economic diversity.
When?
Patient enrollment: Participating ICUs will screen and collect data for up to 20 mechanically ventilated patients per arm admitted to their ICU from the 1st October 2019 until the 1st January 2021. Recruitment time frame could be extended.
Data collection: Extension of data collection to 31st March 2023.
COVID-19: Centers may not be able to engage in clinical research at this time. After finalization of the DTA and IRB approval, individual centers can decide to proceed or to defer data collection, depending on their clinical challenges and availability of personnel for the data collection.
What data is required?
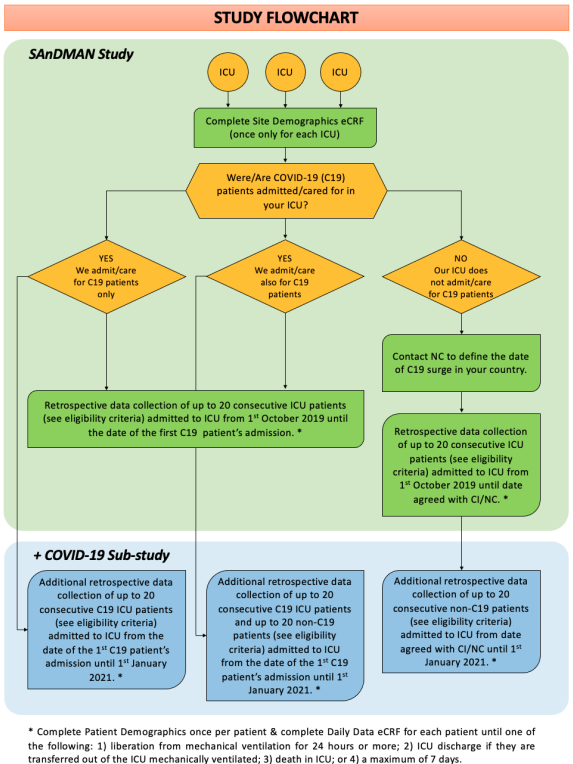
Do I need IRB approval?
Ethics committee and appropriate local regulatory approvals must be obtained by each National Coordinator (NC) /Principle Investigator (PI). As SAnDMAN is a retrospective no-risk study, a waived informed consent model will be used.
How is the data that is collected managed?
All data is anonymised and cannot be linked to individual subjects. The data will be stored securely and all procedures regarding data management will comply with General Data Protection Regulation (GDPR) 2016/679/EU. The eCRF platform is licensed from ClinFile and administered by ESICM.
The eCRF platform is GDPR compliant.
Who owns and can access the collected data?
Each site investigator is responsible for their own data, and may request an export of their locally collected data after the SAnDMAN database is locked. The request should be addressed to the Principal Investigators.
After the primary SAnDMAN manuscript is published, investigators may publish their local data.
Please read the authorship and publication document.
Is there any financial compensation?
There is no financial compensation for participation. Participation in the trial is completely voluntary.
How do I participate?
Registration through the ESICM platform is closed.
SAnDMAN Executive Committee Members

SAnDMAN Steering Committee Members
Geert Meyfroidt MD, Giuseppe Citerio MD, Dylan deLange MD, Ib Jammer MD, Fabio Silvio Taccone, MD Björn Weiss MD, Jorge Salluh MD
Any further Questions?
Please read Frequently Asked Questions (FAQs) document.
You can contact the ESICM office: research@esicm.org and/or sandman.oxford@gmail.com
Documents
- Protocol (update: 19 December 2022)
- Center Form
- eCRF
- Screening Log
- Manual of Operations
- FAQs
- Authorship and publication
- National Coordinators List
- Data Request Form
- Lay Summary
- Trials.gov details