Presidents’ Ground Breaking Research Release
PRESIDENT’S SESSION
Presidents’ Ground Breaking Research Release
 #LIVES2018
#LIVES2018
ESICM President Massimo Antonelli and Jozef Kesecioglu chair this first clinical trials session featuring a selection of the newest ideas and developments in clinical practice at the bedside. This session is not to be missed…
Watch the full session here!
Featured presentations:
The TARGET Trial
 The TARGET trial is a 4000 patient, multi-centre, randomised, controlled, clinical trial designed to determine if increased calorie delivery using an energy-dense enteral nutrition formula to deliver full-recommended calorie requirements, compared to standard care, results in improved patient centred
The TARGET trial is a 4000 patient, multi-centre, randomised, controlled, clinical trial designed to determine if increased calorie delivery using an energy-dense enteral nutrition formula to deliver full-recommended calorie requirements, compared to standard care, results in improved patient centred
 outcomes. Patients were randomised from 46 sites in Australia and New Zealand to receive either 1.5kcal/ml enteral nutrition (energy-dense) or 1kcal/ml (standard care) given at the same rate (1ml/kg IBW/h). The protein concentration was the same in the two formulae ensuring that the protein ‘dose’ received was the same in the two groups. The primary outcome was 90 day survival. A number of secondary outcomes will also be presented.
outcomes. Patients were randomised from 46 sites in Australia and New Zealand to receive either 1.5kcal/ml enteral nutrition (energy-dense) or 1kcal/ml (standard care) given at the same rate (1ml/kg IBW/h). The protein concentration was the same in the two formulae ensuring that the protein ‘dose’ received was the same in the two groups. The primary outcome was 90 day survival. A number of secondary outcomes will also be presented.
~ Sandra Peale (upper right) & Marianne Chapman (Upper left)
Randomised controlled trial of protocolised extubation to non-invasive versus invasive weaning from mechanical ventilation in acute respiratory failure: The Breathe Study
 We will present the results of the Breathe randomised controlled trial – an evaluation of the effectiveness of early extubation to non-invasive ventilation with protocolised invasive weaning in adult patients who were difficult to wean. The Breathe trial took place between March 2013 and October 2016 across 41 intensive care units in the UK. 364 patients were randomised with the primary objective of determining the effect of the different weaning strategies on time to liberation from ventilation.
We will present the results of the Breathe randomised controlled trial – an evaluation of the effectiveness of early extubation to non-invasive ventilation with protocolised invasive weaning in adult patients who were difficult to wean. The Breathe trial took place between March 2013 and October 2016 across 41 intensive care units in the UK. 364 patients were randomised with the primary objective of determining the effect of the different weaning strategies on time to liberation from ventilation.
~ Gavin Perkins
Decontamination strategies in Intensive Care Units: A multinational cluster RCT
 Decontamination strategies such as selective digestive tract decontamination (SDD) and selective oropharyngeal decontamination (SOD) are commonly used in the Netherlands. But is selective digestive tract decontamination (SDD) effective and safe in European ICUs with moderate-to-high levels of antimicrobial resistance? Results of the R-GNOSIS ICU trial comparing SDD, SOD and Chlorhexidine 1% mouthwash to standard care in 13 European ICUs.
Decontamination strategies such as selective digestive tract decontamination (SDD) and selective oropharyngeal decontamination (SOD) are commonly used in the Netherlands. But is selective digestive tract decontamination (SDD) effective and safe in European ICUs with moderate-to-high levels of antimicrobial resistance? Results of the R-GNOSIS ICU trial comparing SDD, SOD and Chlorhexidine 1% mouthwash to standard care in 13 European ICUs.
“This now is the largest European multi-centre trial outside the Netherlands on the effectiveness and safety of decontamination strategies in ICUs.”
~ Bastiaan H. Wittekamp
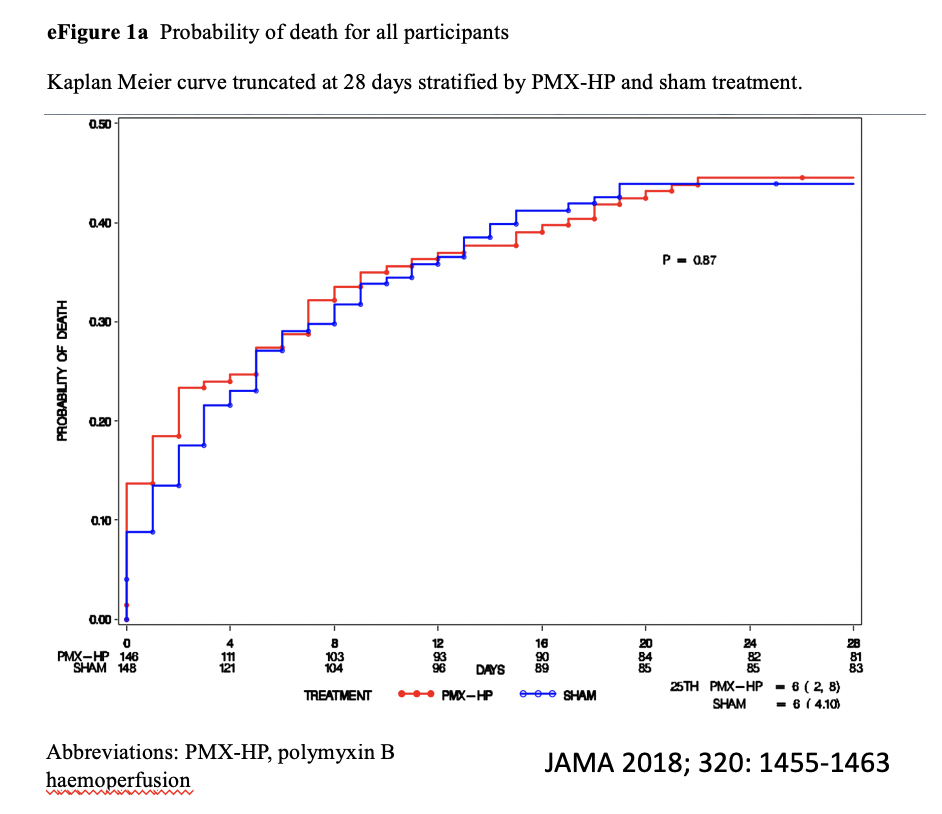
Effect of targeted polymyxin B haemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomised clinical trial
 In this multicentre randomised clinical trial that included 450 adults with septic shock and high circulating endotoxin activity, polymyxin B haemoperfusion compared with sham haemoperfusion did not significantly decrease 28 day mortality, 37.7% versus 34.5%.
In this multicentre randomised clinical trial that included 450 adults with septic shock and high circulating endotoxin activity, polymyxin B haemoperfusion compared with sham haemoperfusion did not significantly decrease 28 day mortality, 37.7% versus 34.5%.
Relevant findings from the trial:
- In patients with high circulating endotoxin activity, polymyxin B haemoperfusion, at the dosing most commonly used internationally, did not affect mortality.
- Polymyxin B haemoperfusion at the dosing used in this clinical trial was not associated with a measurable decrease in endotoxin activity when compared to sham haemoperfusion.
- This study showed that it is possible to effectively blind a large multicentre device trial.
~ Phillip Dellinger
Venovenous ECMO for very severe ARDS: A Bayesian analysis
 In the wake of the publication of the EOLIA trial, the role of early venovenous extracorporeal membrane oxygenation (ECMO) in very severe ARDS remains uncertain and widely debated. We will report the results of a post hoc Bayesian analysis of the EOLIA trial conducted to determine the probability that early ECMO reduces mortality in very severe ARDS.
In the wake of the publication of the EOLIA trial, the role of early venovenous extracorporeal membrane oxygenation (ECMO) in very severe ARDS remains uncertain and widely debated. We will report the results of a post hoc Bayesian analysis of the EOLIA trial conducted to determine the probability that early ECMO reduces mortality in very severe ARDS.
“Bayesian analysis combines the results of a clinical trial with prior information and beliefs (both skeptical and enthusiastic) regarding the treatment effect to produce an updated direct estimate of the probability that the treatment will benefit patients. Bayesian analysis can help to inform the interpretation of study findings.”
~ Ewan Goligher
Full Session Line-up
This session will also include a cast of expert commentators, who will discuss important points following each presentation and open up discussion during this Clinical Trials Session. Here is a full list of clinical trial presentations and commentators:
Global Sepsis Alliance Award 2018
Speaker: Konrad Reinhart, Jena, Germany
ALIVE, the ESICM Fund
Speaker: Martin Dünser, Linz, Austria
Speaker: Mervyn Mer, Johannesburg, South Africa
The TARGET Trial
Speaker: Marianne Chapman, Adelaide, Australia
Speaker: Sandra Peake, Adelaide, Australia
Commentator: Jerry Zimmerman, Seattle, United States
Randomised controlled trial of protocolised extubation to non-invasive versus invasive weaning from mechanical ventilation in acute respiratory failure: The Breathe Study
Speaker: Gavin Perkins, Coventry, United Kingdom
Commentator: Jean-Michel Constantin, Clermont-Ferrand, France
Decontamination strategies in Intensive Care Units: A multinational cluster RCT
Speaker: Bastiaan H. Wittekamp, Utrecht, Netherlands
Commentator: Yatin Mehta, India
Psychological Outcomes following a nurse-led Preventative Psychological Intervention for critically ill patients (POPPI) trial
Speaker: Kathy Rowan, London, United Kingdom
Commentator: María Cruz Martín Delgado, Madrid, Spain
MIND-USA: Phase III RCT of antipsychotics vs. placebo for delirium and other ICU outcomes
Speaker: Wes Ely, Nashville, United States
Commentator: Carl Waldmann, Reading, United Kingdom
Effect of targeted polymyxin B haemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomised clinical trial
Speaker: Phillip Dellinger, Camden, United States
Commentator: Diederik Gommers, Rotterdam, Netherlands
INTEREST Trial results
Speaker: Marco Ranieri, Rome, Italy
Ciro Leite Mendes, João Pessoa, Brazil
Venovenous ECMO for very severe ARDS: A Bayesian analysis
Speaker: Ewan Goligher, Toronto, Canada
Alain Mercat, Angers, France
Join this exciting clinical trials session LIVE in Paris…
Thematic Session ~ Presidents’ Ground Breaking Research Release
22.10.2018, 16:00 – 18:15, room PARIS
Or watch LIVE ONLINE HERE!
 #LIVES2018
#LIVES2018